

This charge separation occurs in chemical bonds when two atoms participating in the bond formation have different electronegativity values. In the creation of a bond dipole moment, the electrical charges are separated as partial charges δ+ and δ. In which, δ is the value of charge and d is the distance between two atoms in the covalent bond.
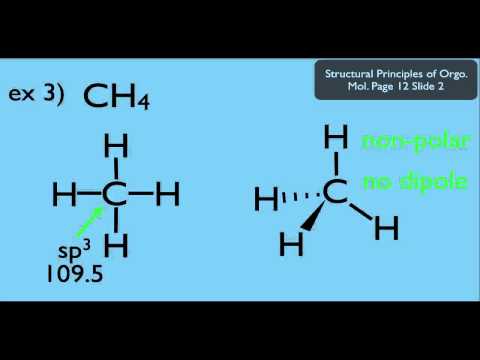
The bond dipole moment is denoted by the symbol “μ”. The bond moment occurs when there is a positive and negative charge separation in a chemical bond. Hence, it gives the polarity of a chemical bond. Side by Side Comparison – Bond Moment vs Dipole Moment in Tabular Formīond moment is the separation of electrical charges in a covalent chemical bond that is present within a certain chemical compound. Similarities Between Bond Moment and Dipole Momentĥ. The key difference between bond moment and dipole moment is that bond moment occurs in a covalent chemical bond whereas dipole moment occurs between two ions in an ionic bond or between two atoms in a covalent bond. Dipole moment, on the other hand, is any kind of electrical separation (separation of charges). It is the polarity of a chemical bond that is located within a certain molecule. The bond moment is also known as bond dipole moment.
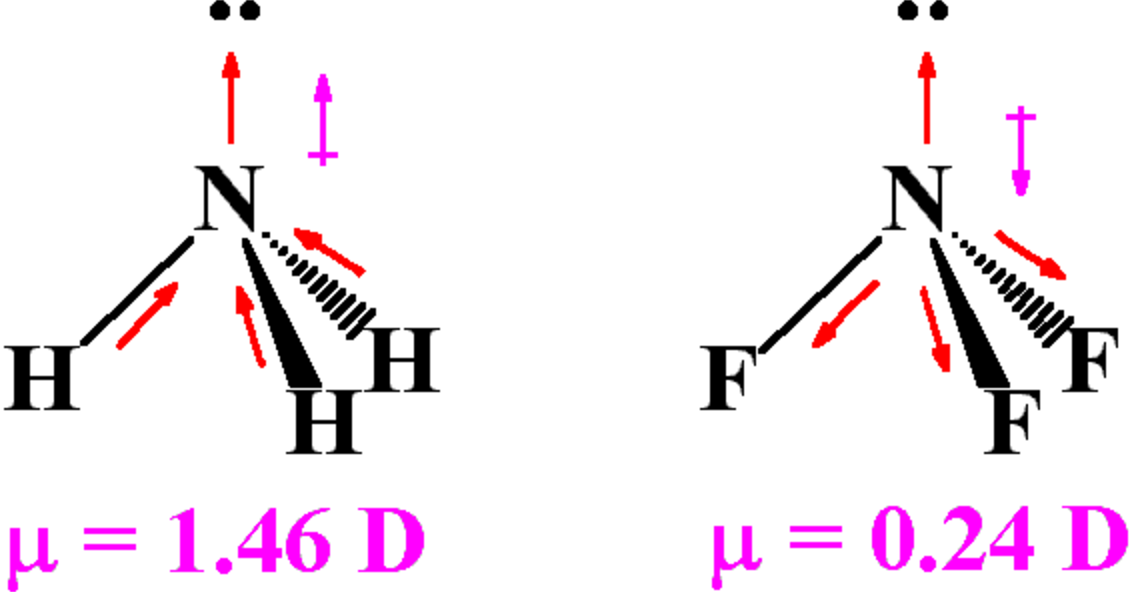
The terms bond moment and dipole moment are based on similar principles but are different based on the application. Key Difference – Bond Moment vs Dipole Moment


 0 kommentar(er)
0 kommentar(er)
